Selected submissions by the class of 2022
Teaching & Learning during COVID-19
As COVID-19 abruptly disrupted our Molecular & Cell Biology II course, we had to adapt. We replaced the second midterm with a free-style piece of scientific communication that they made from home. Submissions included videos, songs, animations, illustrations, poems, and even a puzzle! I am sharing some of the best work from these students -- truly impressive! Congratulations to the entire MCBII class of Spring 2020.
THESE WORKS ARE PROPERTY OF EACH STUDENT.
Precious Adeyemi-Ogunleye
Precious, an incredibly talented singer, tells us in a song all what you need to know about apoptosis. Click in the picture to watch the video in a new page. This is what she said about her video:
"The cell can make life or death decisions. Cells go under different changes such as proliferation and also death. There are so many ways the cell can die. Some ways include apoptosis, necrosis, necroptosis, pyroptosis, ferroptosis, lysosome-dependent cell death and autophagy-dependent cell death.
In my music video, I explained how the cell can die by apoptosis when the caspases is formed intrinsically. Cell apoptosis is a form of cell death that is programmed by the body. It could occur intrinsically(mitochondrial mediated) or extrinsically(death receptor mediated) apoptotic pathways. It causes morphological changes in the cell such as cell shrinkage, membrane blebbing and formation of apoptotic bodies. After the formation of the apoptotic cells, they are engulfed and phagocytosed by the macrophages.
During the formation of the apoptotic cell, Bak or Bax oligomers form pore-like structures in the outer mitochondrial membrane. Cytochrome C in the intermembrane space is then released through this pore into the cytosol. The Cytochrome C activates apoptosome (Apaf-1). Apaf-1 cleaves and activates pro-caspase 9 which triggers a chain reaction of caspase reactions."
Daniel Cho
The super talented Daniel Cho gives us a beautiful and insightful infographic about the actin cytoskeleton. So cool! Everything you need to know is in the poster, so click on it to get it on a better resolution.
Tania Cuevas
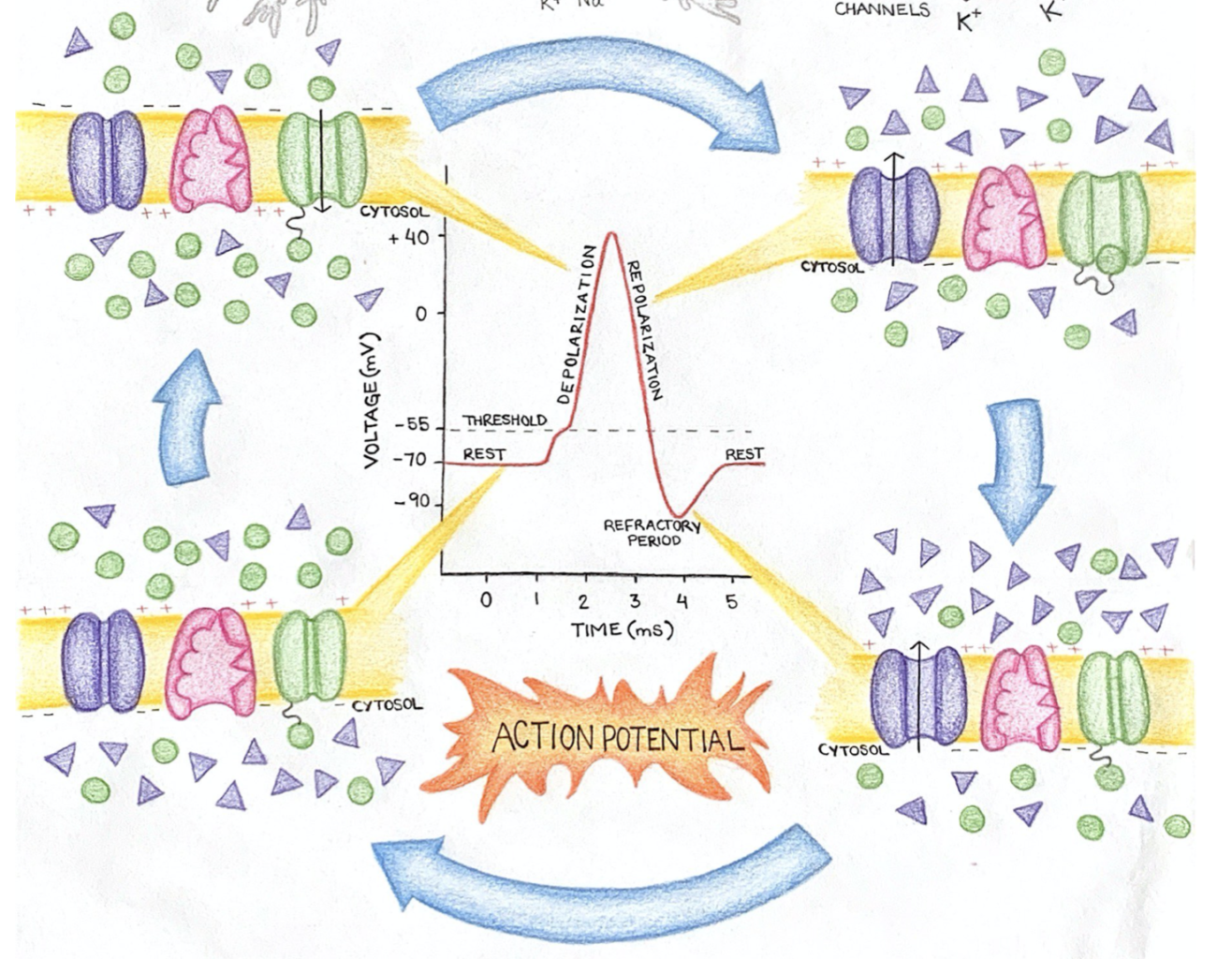
Tankia gives us an amazing animation about the Resting Membrane Potential and Action Potential. In her own words:
"For my creative video, I decided to explain the action potential for an audience that has minimal biology knowledge. I explained in the importance of sodium and potassium ions and channels, and how the membrane potential (-70mV) is due to potassium ions having a greater ion conductance than sodium. I emphasized that membrane voltage is crucial for an action potential to happen and how this membrane resting potential is achieved and maintained by the sodium/potassium pump.
Because I was running out of time, I didn’t get a chance to mention the following key points:
• Glial cells are in charge of myelinating axons. Myelin speeds up the action potential because it prevents it from happening in myelinated regions, so the action potential jumps along the nodes of Ranvier (where there is no myelin).
• The movement of sodium and potassium ions across the membrane during an action potential does not affect the overall concentration of these ions in and out of the cell. The quantity of ions that move might be large, but it is negligible compared to the overall amount of ions present!
• Ions move from high to low concentration without requiring ATP. ATP is used every time an ion is moved across a membrane against its concentration gradient."
Adler Guerrero
The news are calling him the next Liberato Kani. Adler comes with some powerful verses. Rap against cancer! You can learn more about cancer and his lyrics here.
Jennifer Herrera
Do you want to learn about autophagy? Then check Jennifer's awesome animation. It will teach you all what you need to know...
Bahey Abou Hussein
Bahey, also wanted to sing us about apoptosis! Here is his own description:
For this assignment, I parodied "Thank U, Next" by Ariana Grande. I wrote the lyrics, played the music on the piano, and sang the song (poorly). The song is from the point of view of a cell and is primarily about apoptosis. The mechanism detailed in the song is about when a cell has apoptotic triggers and has to commit "cell murder." The cell in the song has apoptotic triggers like damaged DNA and growth factor deprivation. These triggers result in an apoptotic response in which anti-apoptotic proteins are inhibited and apoptotic proteins like Bak and Bax are stabilized. Bak and Bax break down the mitochondrial integrity by making pore-like structures in it. This causes the release of cytochrome c into the cytosol. This cytochrome c activates apoptosome (apaf 1) and is detectable in the first place due to the fact that it's water-soluble. Then apoptosome cleaves procaspase nine and dimers of caspase nine trigger a chain reaction of caspase reactions. After apoptosis is complete, macrophages come to engulf, or "phagocytose," the cell remains. Macrophages know which cells to engulf due to equilibration of PS across the plasma membrane which means there is enough PS on the outside of the cell (due to proteins like Scramblase) to be recognized by macrophages. Finally, apoptosis is completed.
Caroline Lee
All what you need to know about endocytosis is perfectly explained by Caroline's hand-drawn animation. Here is what she tell us:
"Endocytosis is when a cell uses its membrane to encase matter and bring it into the cell in the form of an endosome. Different types of endocytosis include phagocytosis which is specifically bringing in bacteria usually to digest and pinocytosis which is when a cell ingests liquids. There is also receptor mediated endocytosis which uses cargo receptors, adaptin, and clathrin to sense the target proteins and bring them in as needed.
Proteins from the outer cell membrane that need to be degraded are brought in through endosomes for degradation through endocytosis. These vesicles then combine with vesicles from the trans Golgi to form late endosomes. These late endosome are a combination of materials needed from the secretory pathway and the proteins destined for degradation. These late endosomes then later mature into lysosomes which use their acidic environment to break down the proteins so that the cell may recycle the parts. The endosome can also return to the membrane rather than maturing into a lysosome and degrading he membrane and proteins from the plasma membrane."
Sofia Luminari
Sofia gives us a beautiful infographic about the Action Potential. You can download her detailed explanation here.
Abby Niranjan
Abby sing us a beautiful song about Targeting Proteins to the Mitochondrial Matrix. In her words:
"Protein targeting to cellular organelles is a key set of processes within cells, and one process in particular is protein targeting to the mitochondrial matrix. Proteins coded by the nuclear genome and produced by ribosomes in the cytoplasm are typically either directed to the secretory pathway or to organelles including mitochondria, peroxisomes, and chloroplasts. A signal peptide, or targeting sequence, acts as a “zip code” to tell proteins where in the cell they need to go. For proteins that go to the mitochondrial matrix, the signal peptide is located on the N-terminus, consists of 20-50 amphipathic amino acids, is typically not acidic, and is cleaved after the protein reaches its destination. The matrix is an important site for events such as the Citric Acid Cycle, and enzymes necessary for this cycle must be brought into the matrix efficiently. Proteins are imported into the mitochondrial matrix post-translationally, after they have fully been formed in the cytoplasm. Protein import into the matrix costs energy, and this energy is obtained by ATP hydrolysis and energy from the electrochemical gradient created by the proton motive force in mitochondria itself.
Mitochondria consists of an outer membrane, inner membrane, and intermembrane space between the two membranes before the matrix. A key feature of matrix proteins is that they have only one signal that allows them to cross both membranes. First, the chaperone protein Hsc70 (in the cytoplasm) uses ATP to keep matrix proteins unfolded. Only when they are unfolded, they are able to pass through the outer membrane translocon complex (TOM) into the mitochondria. A receptor first recognizes the signal sequence, and then recruits an outer membrane translocon. This in turn recruits another TOM complex, the general import pore, which brings the protein through the outer membrane. Another key feature for matrix import is that the outer and inner membranes come very close together at this step. A translocon complex on the inner membrane (TIM) associates with the protein, and then an Hsc70 chaperone located specifically in the matrix pulls the protein into the matrix using energy from ATP. The final step once a protein has successfully been brought into the matrix is for a protease to cleave and degrade the signal peptide, and for the protein to re-fold."
Kanan Patel
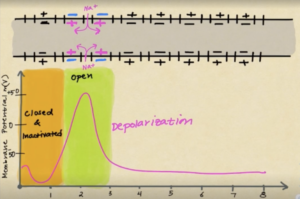
Another super cool video about the action potential! Here is Kanan's explanation:
"Our brain receives signals from all over our body and we have something called a nerve impulse where an electrical signal travels along a neuron. Different ions are continuously moving across the cell membrane, slightly altering the polarity of the cell. An action potential can happen when ions like, sodium and potassium, move across the cell membrane in greater amounts and change the membrane potential; this can be due to something like a slap. The difference in a hard slap or a light slap is the frequency of action potentials: the harder the slap the more frequent action potentials will fire. Normally, at rest, the concentration of sodium ions is much greater outside of the axon than inside; the concentration of potassium ions is greater inside the axon. Furthermore, there are more sodium ions outside the cell than potassium ions inside the cell. The inside of the cell has a high presence of chlorine ions and proteins that cause the overall negative charge of the cell. The cells resting potential is at -70mV and polarized. When the electrical impulse begins, the ligand-gated sodium channels slowly let in sodium, beginning the depolarization of the axon. If enough sodium enters and causes the membrane potential to reach its threshold of -55mV, an action potential will occur; the threshold must be reached in order for an action potential to happen. At this point, the voltage-gated sodium channels will open, resulting in a rapid flow of sodium ions into the axon. This depolarization will bring the membrane potential to around +35mV. Next, the axon will repolarize by the opening of the voltage-gated potassium channels. The potassium ions leave the axon and start to balance out the charges inside and outside of the axon. The voltage-gated sodium channels close. This repolarization will bring the membrane potential back to around its resting potential but the axon will overshoot causing hyperpolarization. The membrane potential will become even more negative than the resting potential. The voltage-gated potassium channels close as well. Now, the cell will go back to its resting potential.
Conduction is to allow the action potential to move down the axon, in segments. When sodium enters an axon and depolarizes it, it will trigger the depolarization of another part of the axon. Next, that part of the axon will become closed and inactivated and hyperpolarization happens to it; the action potential moves along. Once hyperpolarized, sodium can no longer enter there and that area will be closed and back at resting potential. The action potential will progressively move down the axon like this continuously.
Myelination helps speed up the action potential. Myelin sheaths sit on the axons to provide insulation and ultimately gain speed. Between each myelin sheath, there is a node of Ranvier. Action potentials always occur uni-directionally, going from the axon hillock to the axon terminal. And this is how action potential works."
Learta Prishtina
Check Learta's cool animation to teach us about cancer and the tumor microenvironment.
"Cancer is a disease that is caused by uncontrollable cell division. This leads to tumor formation. Tumors can be benign or malignant. Malignant tumors are characteristic of being metastatic, or spreading to other tissues. There are a series of things that make cancer difficult to treat. Some of the challenges consist of a diverse source of causes, its unique ecosystem, the way it progresses and becomes resistant, the different ways drugs effect it, and the immunological effects.
Cancer is caused by a series of intrinsic and or extrinsic factors. Mutation of oncogenes, which promote cellular proliferation or tumor suppressor genes, which inhibit cellular proliferation lead to cancer. Common oncogenes consist of c-src, KRAS, and PI3K. Common tumor suppressors consist of p53, Rb, and PTEN. Mutations of oncogenes or tumor suppressor genes are generally caused by chromosomal translocations, point mutations, and deletions.
Carcinogens are substances that promote the formation of cancer. Common carcinogens are found in cigarettes and processed meats. Like any other ecosystem, the tumor ecosystem constants of biotic and abiotic factors Biotic factors consist of stem cells, epithelial cells, fibroblasts, and immune cells. Abiotic factors consist of cytokines, interleukins, growth factors, and metabolites such as water oxygen and glucose. Something unique/interesting about cancer cells is that they prefer to go through anaerobic respiration over aerobic respiration because of the fast-pace ATP production and CO2 production. This is known as the Warburg effect.
Tumor cells generally accumulate mutations that lead to metastasis. Cancer cells can also develop new proteins. For example, a mutation in the TCA cycle creates a mutated is-citrate dehydrogenase that leads to the formation of histone methyl-transferases and other detrimental to the cells. A series of methods are employed to treat cancer treatment. Common methods consist of using Monoclonal antibodies against tumor cells. Other methods consist of chimeric antigen receptor T cells, vaccines, oncolytic viruses, and immune checkpoint blockades. Some cons of these methods consist of high costs and their efficiency."
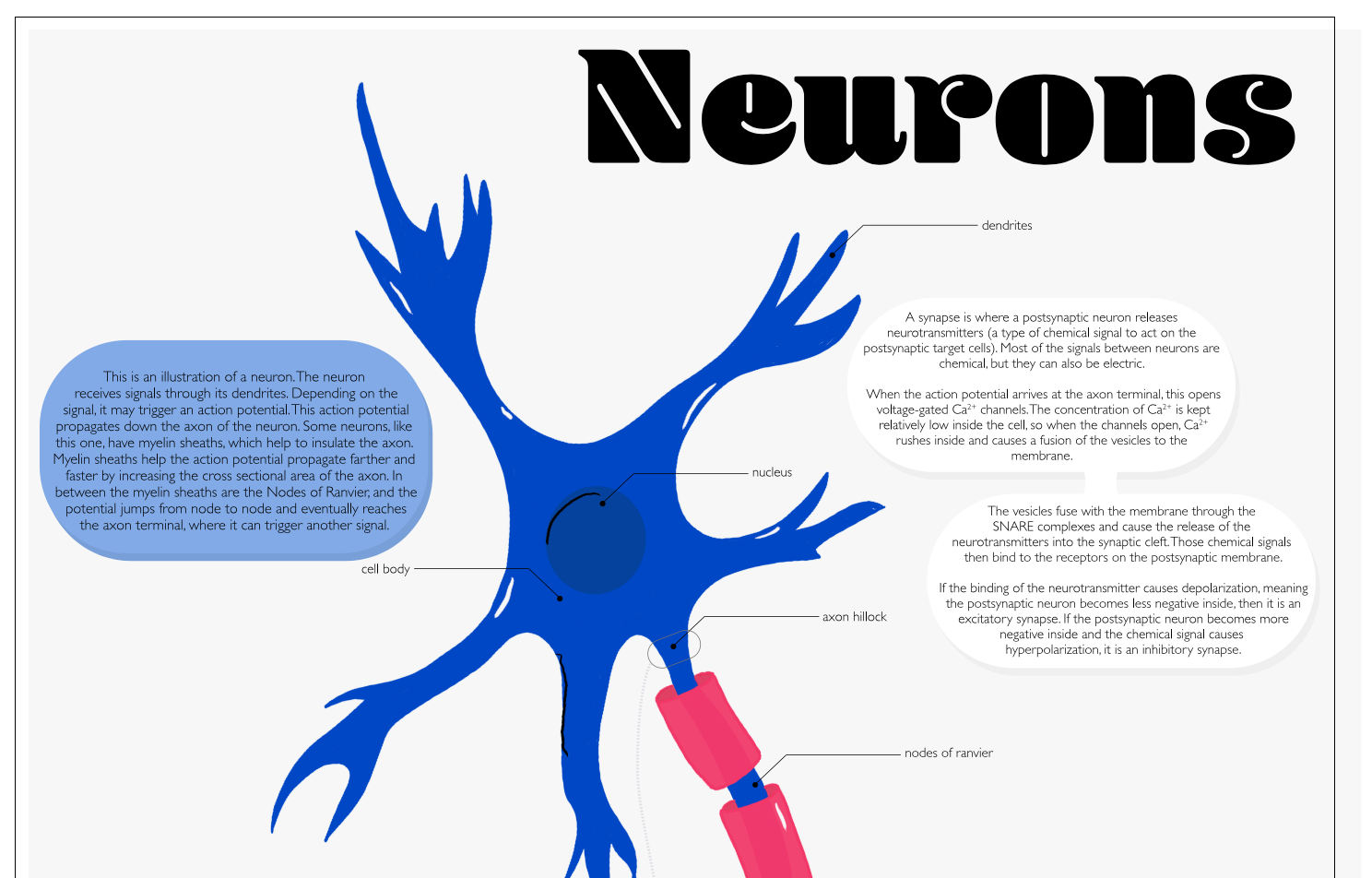
Catherine Ann Quan-Shau
Another beautiful infographic about the neuron. Everything you need to know is in the poster, so click on it to get it on a better resolution.
Olivia Sacco
Olivia made a fantastic child's book about Polly the Polypeptide! Are you stranded at home not knowing what to read your kids anymore? Look no further!
Sonali Sharma
Sonali brings us a beautiful animation about the cell cycle. Lear more with her video and her explanation below:
"There are four main segments of the cell cycle: G1, S, G2, and Mitosis. G1, S, and G2 are collectively known as Interphase. To make sure everything goes as planned during the cell cycle, there are built in check points at various phases. The G1 checkpoint blocks the start of DNA Synthesis. The Intra-S checkpoint prevents continuation of DNA synthesis if the genome is damaged. The G2/M checkpoint blocks entrance to Mitosis if DNA replication is not complete, and the Anaphase checkpoint prevents chromosomes from separating during anaphase if the chromosomes are not correctly assembled.
Let’s take a closer look at how these checkpoints regulate the cell cycle on a molecular level. Cyclins are a class of proteins that control the progression of a cell through dimerization with another class of proteins called cyclin-dependent kinases.
The dimerization of these cdk’s activate them within the cell. Specific CDKs trigger different parts of the cell cycle, and are regulated by phosphorylation. These now active CDKs can phosphorylate other proteins and control their activity.
G1 Cyclins, such as cyclin D, binds with CDK 4 and 6, linking the cell cycle to external cues. Other cyclins bind to other specific CDKs at different stages of the cycle.
Once the CDK successfully phosphorylates the target proteins, the checkpoint is passed and the cell cycle can proceed.
To ensure this system works smoothly and efficiently, cyclins are present in phases. They are produced in the phases leading up to their checkpoint, and once they have been used, their concentration is decreased either by CDK inhibitors such as Rb, ubiquitinated by an APC/C multisubunit complex or taken care of in other ways."
Mohammed Tamara
Mohammed drops us a new tune about how proteins can give you some real bars!
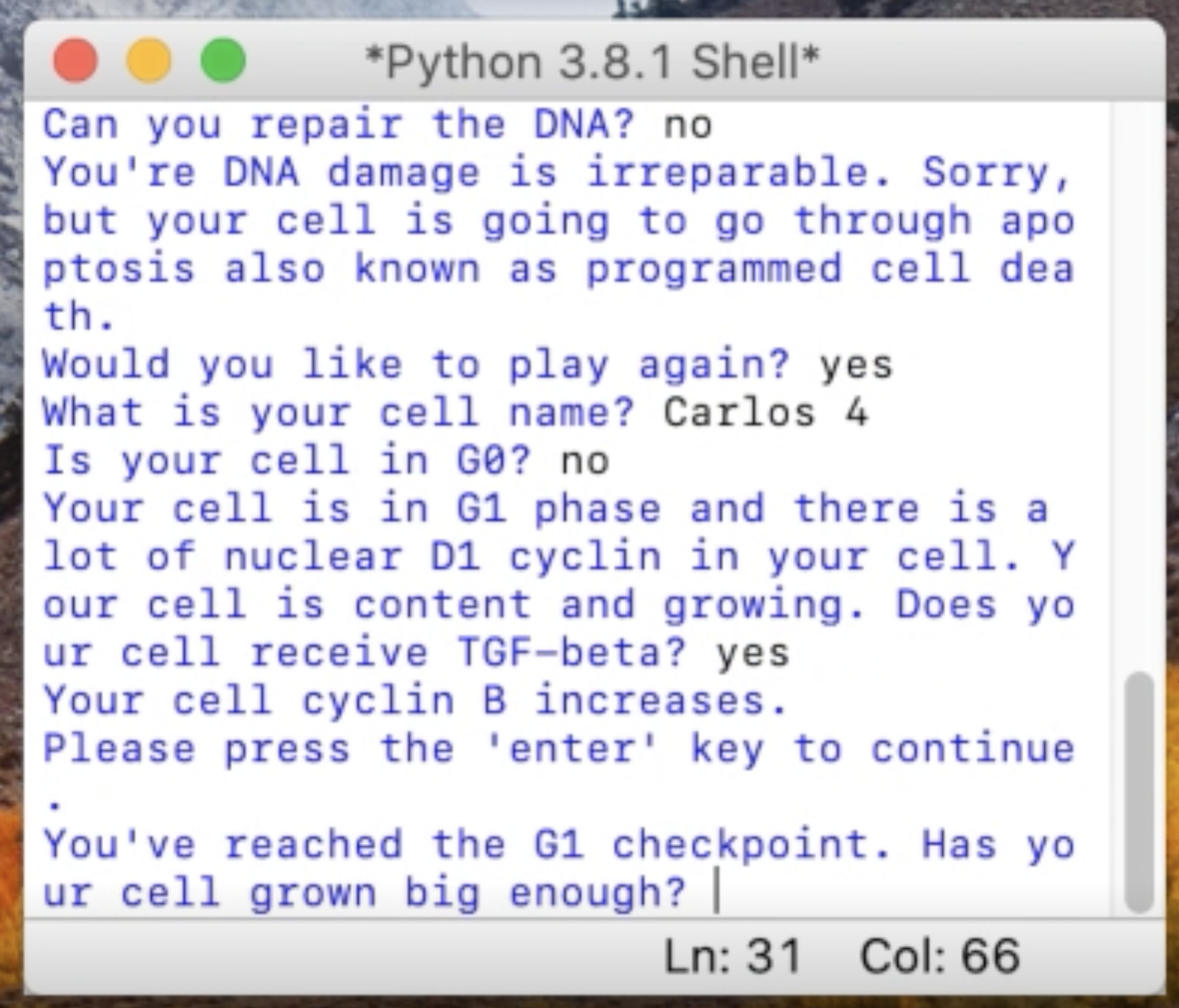
Sandy Yang
Every cells has to constantly make decisions. To illustrate this decision process, Sandy wrote a clever program in Python where a cell needs to make decisions. Be careful, a mistake can be fatal! Learn more about the cells cycle with her explanation of the process.